I have no idea what this paragraph is supposed to mean…
It’s Strong Concordism run riot. Even Moderate Concordism has serious issues; Strong Concordism is completely out of the game.
At the sphere of 100,000 km around Earth2, mentioned in my post on January 12, on average there are no temperature differences. If there would be a difference between position1 and position2 on the sphere, the difference would equalize sooner or later, according the 2nd Law and the underlying principle of Kelvin, respectively Clausius.
Indeed, natural processes as lightning, atmospheric or galactic turbulence, explosions, cooling and heating, tectonic processes, etcetera, can order simple molecules into complex molecules. But these natural processes cannot maintain this order. Snow flakes, sand ribbles, hurricanes, spiral nebulas, crystals, cannot preserve their order and will sooner or later turn again into disorder. The preservation of order requires continual directed effort as predicted by the 2nd Law and the underlying principles of Kelvin and Clausius. This is demonstrated by Miller’s experiment (see my post on January 16, point 9): the transformation of simple substances into amino acids by natural processes stops, unless a factory is built by adding a transport mechanism and safe storage of the amino acids.
You are wrong. The fundamental property of our physical reality, as captured by the 2nd Law and the underlying principles of Kelvin and Clausius for open systems, is that any system ultimately turns into maximal disorder if directed or programmed effort is absent (as a consequence of this, the Circular integral is zero). This fundamental property of physical reality also affects our DNA. Every day, in every cell (including the sex cells), our DNA looses hundreds of thousands nucleotides. Fortunately, most of these mutations are repaired. Without the mutation repair systems in every cell, the DNA would turn into complete chaos within a life time. In 2015 the Nobel Prize in Chemistry was awarded to Thomas Lindahl, Paul Modich and Aziz Sancar for the discovery of these repair systems. A major part of the hundreds of thousands of mutations of the DNA that happen every day in every cell is produced by ‘oxidative deamination’, which makes the letters of the genetic code (A, C, T, G) illegible. This decay process is similar to the oxidation of the ink droplets of a printed text, which becomes illegible sooner or later. Fortunately, the deamination of the DNA is continuously repaired, using the not yet damaged opposite letter at the other DNA strand. The repair starts with the recognition of the damage, followed by a dozen of other steps. In human DNA a total of fifteen proteins are involved in the entire process, which come into action successively.
The vast, daily damage of the DNA in every cell, including the sex cells, is largely the result of the natural oxidation of the DNA. To repair the oxidation, reduction is required. Since the laws of chemistry do not allow oxidation to bring about reduction, mutations can not establish mutation repair.
The theory that mutations can produce mutation repair and that oxidation can produce reduction, is in conflict with empirical science.
my head asplode. Could you walk me through the evidence please?
You can see why this is incorrect if you change the shape of your surface. Your analysis should not depend at all on where you put the surface, as long as it contains only the Earth. So extend it in one direction until it touches but does not contain the sun, and shrink it on the other side so it just touches but still contains the Earth. You now have a standard thermodynamic situation of a system in contact with two reservoirs, one hot (the sun) and one cold (space). Standard analysis of this situation yields the standard answer for Earth’s entropy balance, the one that I’ve previously pointed you to in a published paper.
I will also note that, were your analysis correct, solar energy could do no work, since the outgoing energy from the Earth would have identical entropy to the incoming energy. Solar energy clearly does do work. If your analysis contradicts the standard analysis in the field, and also reaches conclusions that are patently wrong, then you’re doing the analysis wrong.
I suspect I will have nothing further to add here, since the thread has now reached the point of repetition, at least concerning the original claim.
Thank you for this conversation William. We are making some progress here:
Agreement 1) You agree with me that all kinds of natural processes are able to produce “temporary order” by assembling simpler molecules into more complex molecules. That is already a huge breakthrough in our conversation (this is the first part I highlighted in bold above).
Agreement 2) I suppose you would also agree with me that assembled factories and machines function by natural processes. So natural processes can perpetuate molecular “order” if they are channeled by some sort of microscopic machine (this is the second part I highlighted in bold above).
Now let’s zoom in on our Agreement 1. Suppose that over thousands or even millions of years, one of these temporary complex molecules turns out to function a bit like a machine that perpetuates order. Now, according to our Agreement 2, such a machine, once assembled, can function entirely through natural processes. You see, this is how we could travel from simplicity to self-perpetuating complexity, completely through natural means!
According to this dual-stage argument, natural processes can be sufficient to kick-start and perpetuate the (self-)assembly of complex molecules. Is it plausible that such “machine-like” molecules could emerge on basis of our Agreement 1? The current trends in science seem to indicate a loud “yes”! For example, scientists have already discovered relatively simple molecules that are able to produce copies of themselves. See this super interesting paper from Nature:
http://www.nature.com/nchem/journal/v8/n3/full/nchem.2419.html
It was published by a team of chemists from the University of Groningen. Starting from simple molecular building blocks, they observed the spontaneous emergence of such a self-replicating molecule. Based on this “ancestor”, their laboratory environment soon gave rise to a new “species” of these molecules which competed with its ancestral species for building blocks (“food”). These molecular replicators were found to counteract the thermodynamical preference of the system. I quote from their abstract:
“The results show that the second replicator set is a descendant of the first and that both sets are kinetic products that oppose the thermodynamic preference of the system.”
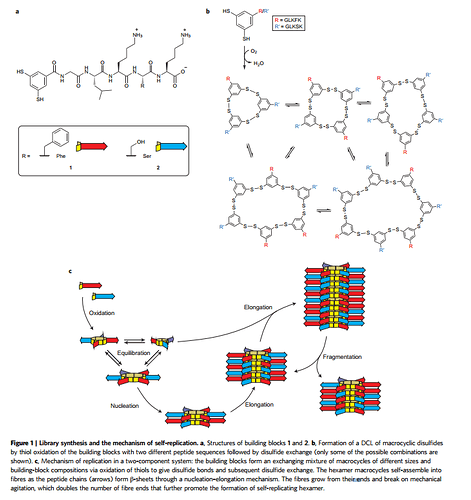
How simple are these self-replicating structures? Basically they are just rings that “like” to be stacked on top of each other, until the structure becomes too long and breaks into two separate structures (creating “offspring”). It’s worthwhile to reproduce the figure from that paper here to show how simple these molecules are:
I have built this argument entirely on our Agreements 1 and 2. I find that the formation of temporary complex molecules (Agreement 1) is a plausible natural pathway towards small molecular machines that counteract the thermodynamic preference of the system (Agreement 2).
Vanity of vanities, says the Preacher,
vanity of vanities! All is vanity. Ecc. 1
Who would have thought that the Preacher was an expert in thermodynamics.
No ‘directed or programmed effort’ violates thermodynamics. In fact, the implementation of ‘directed and programmed effort’ relies on coupling physical processes that result in a net negative ΔG (Gibbs Free Energy). One can’t discuss how local changes in thermodynamic entropy may occur without considering net energy flow and distribution of specific systems.
Simply put, there is no thermodynamic theory of ‘intention or programmed effort’ that is distinct from basic thermodynamics.
You are claiming that mutating replicators are thermodynamically unfavored to persist over geologic timescales. Show your work. For example, calculate the approximate entropy difference between humans and great apes. Calculate the net ΔG for the evolutionary processes (replication, drift, selection & etc.). Is it positive or negative?
Crystals? In that case you should send me all the gemstones (such as emeralds) in your house, so I can dispose of them properly. I’d hate for one of them to turn into disorder and hurt you.
(You’re welcome.)
In most of your posts you stridently remind us all that what was emerged as organized and complex will all eventually crumble into disorder.
My dear sir, SO? Nobody has ever said that animals that evolve will not die… or conversely that the complexities of a beehive are eternal.
Even somebody as complex as Einstein - - still submitted to disorder and death.
What do you think you need to prove to us? We all get it. And it pretty much has nothing to do with Evolutionary theory being right or wrong.
Do we say: that CANNOT be a cup of coffee … because matter is corruptible?
Natural processes are decay processes. They cause hundreds of thousands of mutations every day in every cell. Mutations are the cause of cancer and hereditary diseases. Fortunately mutations are repaired by mutation repair processes, which are present in every cell. In my post on January 16, I remarked: “The theory that mutations can produce mutation repair and that oxidation can produce reduction, is in conflict with empirical science”.
Clarification:
(1) Gravity (attraction between masses) cannot produce Gravity-Reverse (repulsion between masses)
(2) Rust of an iron plate (oxidation) cannot produce Rust-Reverse (reduction)
(3) Decay of the letters on a printed sheet of paper (fading of the print) cannot produce Decay-Reverse (the restoration of the illegible words and letters).
(4) Decay of the nucleotides of the DNA (mutation) cannot produce Decay-Reverse (the restoration of the illegible words and letters).
These 4 impossibilities follow from the fundamental characteristics of our physical reality and the laws of empirical science. If you want to deny these four impossibilities, you have to provide empirical evidence of a process M that produces at the same time M-Reverse.
I investigated the behavior of Earth2 (identical to our earth, except the presence of living organisms) after placing it in the free continual energy of the Sun at the position in the universe of our Earth. I drew a virtual sphere with a diameter of 100,000 km around Earth2 as its boundary. At this boundary, no influences from the atmosphere of Earth2 are present. Because Earth2 is about 150 million km away from the Sun, the radiation of the Sun at the boundary of Earth2 is uniform, averaged over a few years. I proved that no differences of temperature T are present at the boundary of Earth2. As incoming and outgoing energy flows are equal on average over a few years, the circular integral of dQ/T over the boundary of Earth2 during these this period of time is zero. According the 2nd Law, the disorder at Earth2 increases. I illustrated this theoretical analysis with an experiment, consisting of a rock hanging in the light of a strong lamp sprayed with the fluid Miller used in 1953 in his amino acid experiment. I did not observe molecules to order themselves ever further at a certain position of the rock in combination with molecules falling apart in a higher degree at another position of the rock. No ‘Flintstone battery’ emerged.
You chose an alternative boundary for Earth2, by stretching the sphere around Earth 2 at the side that faces the Sun almost to its surface. As a consequence, the Sun’s radiation over this alternative boundary is no longer uniform, and temperature differences are present at the alternative boundary. As a consequence, the calculation of the circular integral of dQ/T over the alternative boundary over a few years is very complex. You claim that your calculation shows that energy is captured inside the system, resulting in a decrease of entropy. The outcome of my experiment with a wetted rock positioned in free flowing continual radiation contradicts your calculations. Please provide empirical evidence that putting a wetted rock in the sunlight can produce a ‘Flintstone battery’. Such evidence would be breaking news, because it would make an end to the industries that produce solar panels and Lithium batteries.
You are wrong. In post1 of this thread I wrote: “Complex molecules that are produced from simple molecules by natural processes as lightning, wind, rain, heating, cooling, radiation, tectonic forces, etcetera, will fall apart sooner or later; the larger the molecules that are formed, the sooner”.
The research group of Sijbren Otto (University of Groningen, The Netherlands) has conducted the following experiment. Firstly an amount of highly active building blocks was produced in an ‘active building blocks factory’. The building blocks were poured in a cylinder containing a suitable reagent. Hereafter, the highly active building blocks started to ‘join hands’ and formed rings of 12 or 13 building blocks. Also the rings are highly active and started to form strands of rings. The top and tail of each strand is the coupling point for another active ring. As a consequence, the strands continued to grow in length, competing with one another for new rings to be attached. The process slowed down when the active building blocks and rings became scares. Finally the process stopped when all building blocks were coupled in a ring or as a ring in a strand. By shaking the cylinder, the reaction between the active building blocks could be acccelerated, by making the strands break, creating new coupling points. In advanced experiments, several variants of active building blocks are made and competition between variants of rings and strands can be observed in the cylinder (compare the competition between 4 variants of digital amoebae for digital food, I conducted : http://benthamopen.com/contents/pdf/TOEVOLJ/TOEVOLJ-5-1.pdf ). The experiments of Sijbren Otto are claimed to prove the existence of self replicating molecules. But there is nothing more to see than an ordinary chemical reaction between highly active building blocks that are produced in a chemical factory.
This was the dream of the Alchemists. They are back!
Physical processes never violate Thermodynamics, as the laws of Thermodynamics describe how physical processes run through time. Theories, in contrast, can violate Thermodynamics if they make claims that are in contradiction with the laws of Thermodynamics. For instance the theory that putting a wetted rock in the free flowing radiation of the Sun will turn it into a battery; or the theory that simple molecules will order themselves into ever more complex molecules and maintain this order themselves.
Crystals of ice, salt and sugar will fall apart sooner, than crystals of carbon. Nevertheless, decay is the natural course of events. We have to live with it.
My aim is to make clear that the second Law of Thermodynamics holds for open systems. The 2nd Law captures the fundamental characteristic of our physical reality that natural processes are decay processes. A theory that claims that decay processes can repair and innovate is in conflict with empirical science.
I think you’re being overly antagonistic here. I was already talking about “temporary” order to incorporate your point of decay happening “sooner or later”.
If you read my post again, you’ll see that I didn’t quote that research as “proof”, but rather as an “indication” of how simplicity can lead to self-perpetuating complexity. Take another look at figure I posted in my previous reply. It illustrates the simplicity of those “highly active” chemical building blocks.
If such simple structures can already be “highly active”… Imagine all the possibilities that could be explored on a “laboratory” the size of the Earth over the course of millions of years. Put frankly, given all the processes you have already conceded here, I don’t see the problem at all.
Believing in the possibility of some natural occurrence (such as abiogenesis) is different from actually trying to orchestrate it ourselves. But, in principle, I’m open to that becoming a possibility in the future. Why not?
Your comparison with Alchemists is unfortunate because they were very ill-informed in their pursuits and combined it with all kinds of occult practices.
This is a very odd sentence. What does it mean? Later you cite gravity as an example. Are you saying gravity is a “decay process”?
What mutations can produce and what oxidation can produce are two very different subjects. The fact that you are performing this kind of equivocation demonstrates you are indulging in rhetoric rather than practicing science.
But this tells us nothing about evolution. It certainly does not provide any evidence against evolution. It definitely does not provide any argument against the empirically established fact that mutations can provide advantages to an organism and species.
Seeds sprouting are natural processes but are not decay processes.
And yet … in a nuclear charge with exactly known radioactive components … when a nuclear explosion is triggered… VERY SPECIFIC conversions of known matter is unleashed. And the exact nature of remnants can be measured and identified. The MISSING matter, when mathematically converted to energy, reflects the actual explosive energy generated by the bomb.
Measurements like this makes it possible to calculate what kinds of very SUN energy is required to CREATE new elements out of IRON in the ga-billion stars in the Universe.
This is not consistent with a SIMPLISTIC rendering of the 2nd Law of Thermodynamics. How can we get ever-more complex molecules (with very elaborate electron clouds being established as the molecule’s nucleus is forced to grow larger) … from the chaos of swirling energy, atomic fission and atomic fusion?
And yet … it happens throughout the Universe!
The experiment of Sybren Otto does not show simple molecules start ordering themselves into highly active building blocks, which subsequently form rings and strands. The highly active building blocks are produced in a factory, in advance of the experiment. The experiment actually starts with pouring these highly active building blocks in a cylinder where they begin to form rings and strands. By stirring the content of the cylinder, the chemical reaction is intensified. After a while the chemical reaction ends. Some days or months later, the strands and rings in the cylinder begin to fall apart. No self maintenance or self replication can be observed.[quote=“Casper_Hesp, post:52, topic:26534”]
Believing in the possibility of some natural occurrence (such as abiogenesis) is different from actually trying to orchestrate it ourselves. But, in principle, I’m open to that becoming a possibility in the future. Why not?
[/quote]
Never, in any laboratory in the world, it will be found that simple molecules start ordering themselves into ever more complex structures, and start to maintain their complexity and expand it ever further. Such an event would mean that energy becomes available for free. Regrettably, miracles only happen in fairy tales or in the dreams of the Alchemists. (Notice that an increase of complexity of a molecule corresponds with a higher energy content of the molecule).
Your question “Why not?” shows that the Alchemists are back and that the laws of empirical science have lost their authority.
When I look into a mirror or to my house or my car, it is clear to me that gravity causes decay. Nobody has ever observed that gravity produces repair or innovation. Or do you have empirical evidence of such a thing?
(1) Decay is the natural course of events. The 2nd Law captures this fundamental characteristic of our physical reality.
(2) Natural processes shape the natural course of events.
(3) Natural processes are opposed to industrial processes, which are make or maintain processes and are found in factories. Miller’s two experiments (see my post nr. 28 on January 17, point 9) clearly illustrate the differences between a natural process (e.g. lightning, gravity, radiation, tectonic activity, atmospheric activity) and an industrial process.
You are wrong. The natural course of events (as captured by the 2nd Law) is decay. A theory that claims that decay can repair decay (!) and can produce innovations (!) is diametrically in conflict with empirical science. Therefore, evolutionary theory must be formulated more accurately. This need can be specified in more detail:
(1) Living nature continuously adapts to changing circumstances by the mechanism of recombination of alleles and by gene regulation. So, evolution exists (don’t worry). But it is an empirical fact that mutations of the DNA do not improve the DNA and expand it ever further with new functionalities. Instead, mutations are the cause of cancer and hereditary diseases. According to the playing rules of empirical science, the theory that mutations and a dysfunctioning mutation repair system are the motor for improvement of the DNA and expansion of it with new functionalities, must be rejected.
(2) The theory that organic molecules have an intrinsic desire to order themselves into ever more complex structures is based on the false experiments of Miller. Billions of tons of primordial soup can only be produced by building a factory, as Miller proved.
(3) Molecules do not start to order themselves into ever more complicated structures, as the experiments of Sybrand Otto prove.
(4) Mutations cannot produce mutation repair processes.
You are trapped in circular reasoning, by presenting the existence of living organism as proof that natural processes can produce living organisms. [quote=“gbrooks9, post:55, topic:26534”]
How can we get ever-more complex molecules (with very elaborate electron clouds being established as the molecule’s nucleus is forced to grow larger) … from the chaos of swirling energy, atomic fission and atomic fusion?
And yet … it happens throughout the Universe!
[/quote]
Indeed, by the natural processes in the kernel of stars, or by the explosion of stars, or by collision of simple atoms or molecules after being hit by high energy radiation, more complex atoms or molecules can emerge. But natural processes cannot maintain these more complex atoms or molecules and cannot expand their complexity ever further, as Miller found in his experiment in 1953 (see my post nr. 28 on January 17, point 9). Miller proved that a factory must be built to protect and maintain the amino acids that are formed by a natural process as lightning.
The Alchemists believed that matter contains a hidden power to order itself into ever more complicated and valuable structures. Empirical science disproved this belief ages ago. Regrettably, this belief is back, and is sold as science.
Exceptions to everything. One could say that gravity makes pressure and creates rocks and gemstones. And the gravity of stars is essential to the production of just about everything.
Not sure what your thinking is here. I’m just pointing out a natural process that is not a decay process.
@WilliamDJ, I have high hopes for you - - but not if you are going to throw yourself on your sword trying to prove the 2nd Law of Thermodynamics makes all other processes impossible.
Didn’t you already accept that nuclear fusion within a star, which is triggered by the massive pressures of gravity created by the aggregation of helium and hydrogen in the middle of the vacuum of space, is what creates new and heavier elements (elements like iron) ?
Fortunately for humans and life in general, the cycle of existence for star formation, followed by star self-obliteration, is billions of years … which is more than enough time to create life and even celebrate life, no matter which way God decides to go about it!
Hi William,
There are plenty of experiments that demonstrate increasing order in an arbitrarily defined space (for example, inside a flask). When that happens, however, there is a related increase in entropy somewhere else that is greater than the entropy decrease inside the arbitrarily defined space.
Similarly, there can be a decrease in entropy on the earth (an arbitrarily defined space) that is not miraculous. Instead, it is related to an increase in entropy somewhere else (the sun).
The point I just made has been stated many, many times in the thread. You keep stating that those who accept the theory of evolution are doing so in defiance of the 2d Law. They are not; they recognize that increases in solar entropy outweigh decreases in entropy on the earth.
Best regards,