Thank you for this conversation William. We are making some progress here:
Agreement 1) You agree with me that all kinds of natural processes are able to produce “temporary order” by assembling simpler molecules into more complex molecules. That is already a huge breakthrough in our conversation (this is the first part I highlighted in bold above).
Agreement 2) I suppose you would also agree with me that assembled factories and machines function by natural processes. So natural processes can perpetuate molecular “order” if they are channeled by some sort of microscopic machine (this is the second part I highlighted in bold above).
Now let’s zoom in on our Agreement 1. Suppose that over thousands or even millions of years, one of these temporary complex molecules turns out to function a bit like a machine that perpetuates order. Now, according to our Agreement 2, such a machine, once assembled, can function entirely through natural processes. You see, this is how we could travel from simplicity to self-perpetuating complexity, completely through natural means!
According to this dual-stage argument, natural processes can be sufficient to kick-start and perpetuate the (self-)assembly of complex molecules. Is it plausible that such “machine-like” molecules could emerge on basis of our Agreement 1? The current trends in science seem to indicate a loud “yes”! For example, scientists have already discovered relatively simple molecules that are able to produce copies of themselves. See this super interesting paper from Nature:
http://www.nature.com/nchem/journal/v8/n3/full/nchem.2419.html
It was published by a team of chemists from the University of Groningen. Starting from simple molecular building blocks, they observed the spontaneous emergence of such a self-replicating molecule. Based on this “ancestor”, their laboratory environment soon gave rise to a new “species” of these molecules which competed with its ancestral species for building blocks (“food”). These molecular replicators were found to counteract the thermodynamical preference of the system. I quote from their abstract:
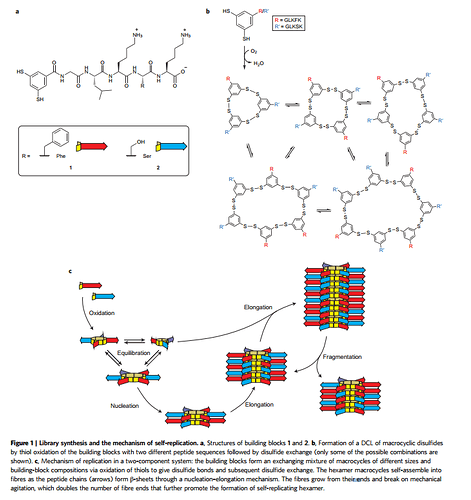
“The results show that the second replicator set is a descendant of the first and that both sets are kinetic products that oppose the thermodynamic preference of the system.”
How simple are these self-replicating structures? Basically they are just rings that “like” to be stacked on top of each other, until the structure becomes too long and breaks into two separate structures (creating “offspring”). It’s worthwhile to reproduce the figure from that paper here to show how simple these molecules are:
I have built this argument entirely on our Agreements 1 and 2. I find that the formation of temporary complex molecules (Agreement 1) is a plausible natural pathway towards small molecular machines that counteract the thermodynamic preference of the system (Agreement 2).