I believe that this is because they are only getting the information from The old Earth scientists and not from ones who actually studied it from the god point of view which as Christians is the only point of view they should ever listen too
I understand how uranium decay works. But if an element is uranium right now, it’s uranium, not lead, so it is impossible to take the element uranium out of the earth and find it is half lead. They are different elements. It takes 4.5 billion years for half a sample of uranium to decay into lead. At that point, you could still take uranium out of the sample that would be pure uranium. When uranium is mined it is extracted from the other elements around it by chemical leaching, so what is mined is just the uranium, not the other elements. Are you claiming that when rocks containing uranium are mined, those rocks are 99% uranium and less than 1% lead is present? Where did you get that information?
Okay you’re starting to get it. When they mine uranium out of the ground it comes out of shelves of uranium not just little granules but like coal and if Earth was 4.5 billion years old those shelves would be 50% uranium 50% lead. Half of the entire shelf would have decayed into lead. Even in the granule uranium this would be the same after 4.5 billion years it would be 50% lead and the other half would be uranium if Earth was 4.5 billion years old.
I’m not a geologist, and I don’t know everything there is to know about uranium ore deposits. But I do know that rocks are constantly being melted and broken down and elements in rocks are constantly being leached out as they interact with the elements found in air and water and form compounds. Uranium is mined from granite, sedimentary rocks and soil, and volcanic rocks. Geology of Uranium Deposits - World Nuclear Association
There is no such thing as a “shelf of rock” that has existed in the same place and same form since the formation of the earth where one would expect to find a sample of uranium that had been left to decay undisturbed for 4 billion years. Rocks that were formed in the Hadeon Eon are extremely rare and are found on the surface in only a few places on earth. Most rocks on earth have been melted or broken down by erosion to form new rocks many times over the course of Earth’s history.
When rocks are dated with uranium, it’s only a specific kind of rock, usually ones that contain zircon crystals. Zircon crystals do not change as they are heated in the rock cycle and the physics of zircon crystal formation resists the incorporation of lead when the crystals are forming but does incorporate uranium. So all lead found in zircon crystals comes from the decay of the incorporated uranium, and we can date the formation of the zircon crystal using the known half-life of uranium.
Most of the granite that is mined for uranium was formed in the Jurassic or Triassic 250-130 million years ago. That period of time is only 5.6-2.9% of uranium’s half-life, so you would not expect 50% of the uranium in those deposits to have decayed to lead. https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/116/5/1115/595088/Release-of-Uranium-from-Uraninite-in-Granites?redirectedFrom=fulltext
You do not have to be an atheist to disagree with creationist nonsense; most Christians I know who work in science do not at all identify as “creation scientists”, and generally accept the mainstream narrative of cosmology, geology, and evolution. Christians who pursue the sciences tend to agree with other scientists because they actually work with the evidence, which speaks for itself.
Actually, all uranium mined out of the earth is 100.00% uranium. What makes you think that the same scientists who are clever enough to have developed nuclear weapons at the Manhattan project and Los Alamos, and who design nuclear power plants, are so thick that they cannot see the implications of physics for the age of the earth and become creationists? I would suggest it is because they have a deeper understanding of nuclear decay than you possess, and that leads to the conclusion of an old earth.
That the half life of U-238 is comparable to the age of the Earth simply means that there was twice as much of that isotope around at the origin. There is plenty of lead extent so there is no problem here, and you have debunked nothing.
Greetings and welcome.
Are you sure you don’t just dislike the type of math required to understand God’s universe? It’s truly amazing stuff, isn’t it?
I don’t like math, either; and I tend to feel that way, too. However, just because it isn’t intuitive to some of us without math brains doesn’t mean God didn’t put it there. Thanks.
You might want to check the Wikipedia article on the Shinkolobwe mine, where the ore used for the Manhattan project was found.
From the article:
Note the ore that was dug up was not 100% uranium. In fact it is strangely close to the 50% that an old earth would require but no where near what a 6,000 year old earth would require.
That’s still not right, because as I pointed out above, no matter where the ore is found, it’s not found in 4.5 billion year old rocks. It’s not this undisturbed sample that has just been sitting there decaying since the formation of the Earth.
Hard to really understand what you are saying, be it appears you think the layers of rock should test younger than the fossils they cover. The problem is that nearly all fossils are covered by sedimentary rock, which cannot be dated with the usual dating systems. (When you grind up a volcanic rock that you can date, then press the granules into a sedimentary rock, the granules still date to the original volcanic rock. You can’t restart the clock until you melt it back down, as I understand it). Now, at times you will get a volcanic rock layer somewhere above or below a fossil, which can be used to at least frame the dating of the fossil a little better.
Also, when uranium degrades to lead, it goes though many other elements, one of which is radon, a gas. So, it is not simple change to lead as you imply. I am not a geologist either, but if you learn a bit you will see how your arguments really are not valid.
You are in complete denial of rationality through superstitious terror and your “example” is utterly bizarre. Again what has any of this got to do with the revelation of Love in Christ?
It doesn’t work that way I’m afraid.
The one thing that you need to understand is that science has rules. There are rules that scientists have to follow that have nothing to do with “atheism” but that are concerned with honest reporting and honest interpretation of accurate information – nothing more, nothing less. These are rules that have been forged in the fires of hands-on experience, and that have proven themselves time and time again in situations where getting them wrong would have (and has had) devastating consequences.
In a nutshell, we’re talking about the basic rules and principles of mathematics and measurement.
The reason why “creationist scientists” aren’t accepted by scientists (and that includes scientists who are Christians as well as those who are atheists) is that they don’t follow the rules. Conventional old-earth geochronology and evolutionary biology, by contrast, do.
“Propaganda” has nothing to do with it.
In actual fact, we do find uranium mixed with its decay products (specifically, lead). More to the point, we find lead in places where the only way it could possibly have got there is by decay from uranium. Zircon crystals are an example here: when they are first formed, they can accept uranium into their crystalline structure, but not lead because lead simply doesn’t fit. So if the zircon crystals were only a few thousand years old, rather than billons, the amount of lead that they contain would be negligible. But it isn’t.
@carl_ward’s point was the uranium found is 100% uranium, which of course is false. The uraninite which is mined is only 65 % uranium at best. And yes it is found in rocks that are only 630 Ma old, but he wouldn’t accept the dating of the rocks. It is a little harder to ignore the simple percentage of the rock which can be measured by any creation scientist who could chose to do so.
U-238 has a half life of ~4.5 billion years. That means half of the U-238 will decay to lead in 4.5 billion years. After 4.5 billion years, the half that has not decayed is still 100% U-238.
The other naturally occurring isotopes have much shorter half lives, and wouldn’t you know it, they are less abundant compared to the longer lived isotope.
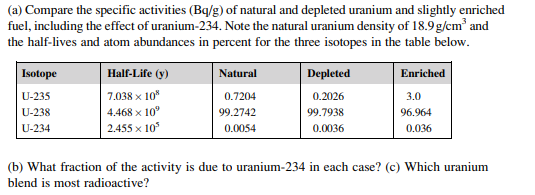
If you can understand the following, it should help you get the big picture about radionuclide decay rates (it is not about radiometric dating of objects on the earth, it is about extinct radioactive atoms):
Radioactive Atoms — Evidence about the Age of the Earth - Ken Wolgemuth
Some evidence on many of the topics raised here from my own field of expertise:
Speed of deposition:
All off the southeastern US marine deposits requires a few centuries at an absolute minimum to form (given some unrealistically high estimates of population density), given the lifespans of the organisms involved (I have counted the growth rings in large Ostrea compressirostra and found them to be close to 50 years old at death, and a large Chesapecten jeffersonius can be 80).
There is completely unambiguous evidence of a sequence of layers: many are unconsolidated or partially consolidated, and hence cannot have been rotated. Most also have index fossils which are only known from that single formation, and no other, which makes a stack of twenty formations certainly appear to require far longer than six months.
Also, almost none of the layers in the Carolinas can be radiometrically dated, due to a lack of volcanic ash. A number of the Floridean formations can be, and given the obvious similarities between certain Floridean deposits and more northerly ones, one can infer age-equivalence.
Glenn Morton, who we lost not too long ago, had a great article on this subject. Below is just one section, and it deals with crinoids.
Crinoids are these small creatures:

Obviously, it takes some time for these creatures to live, die, and then accumulate into a deposit 2,000 feet thick.
Order of magnitude, something like 10,000,000,000,000,000,000 crinoids.
This topic was automatically closed 6 days after the last reply. New replies are no longer allowed.